
Nickel is a metal element in nature with a shiny , silvery appearance and is the fifth most common element in the earth .
Nickel ( NI ) is a metal element , with the atomic number 28 and capacities 2 and 4 , in white , polishes , with crystalline structure and shape . It is flexible , hard and conductor of electric currents in the group VI and in 4th period of the periodic table . The atomic mass of nickel is 58.71 and has five stable isotopes .
Nickel is a metal element in nature with a shiny , silvery appearance . It is the fifth most common element in the Earth , and is abundantly available in the Earth 's crust and the Earth 's core . Along with iron , it is a common element in the meteor and can even be seen in small quantities in plants , animals , and sea water .

The Earth's core is basically made up of nickel and iron alloy . Nickel alloy is a part of the group of iron and cobalt elements and is similar in terms of magnetic properties and chemical activity .
Nickel alloy is not oxidized because it has a lot of resistance in the air and in the water ; for this reason , it is used for producing currency coins , metal working of brass and iron as well as for making instruments in particular alloys such as German silver .
Nickel producers in the world are Russia , Australia , and Canada , respectively .
Nickel has been found in ancient metal objects dating back to more than 2,000 years . Axel Fredrik Cronstedt , Swedish chemist , identified it as an element for the first time in 1751 . The use of nickel became more and more in 19th century because of using it for metal plating in alloys like ‘’silver – nickel ‘’ (German silver ). [ The alloy is named for its color , otherwise there will be no silver in it ] .
In the fifteenth century , German miners found a red - brown mine that believed to contain copper . They called it “ copper – nickel or evil copper “ because they could not separate copper from it .
First the united states , coined from the brass and nickel alloy in 1857 . At that time , there was still nothing as pure nickel but in 1881 , pure nickel was used for coins in Switzerland .
Stainless steel was discovered in the early 20th century and found that nickel plays a critical role in many different grades of stainless steel .
It has been found that nickel – based alloys have extraordinary resistance to corrosion and can withstand high temperature . This enabled them to be suitable for the use in chemical material manufacturers and allow a jet engine to be practical .
As a result of these developments , nickel was faced with extreme demand in the last century . This situation continues today because of its fundamental role in many technologies .
It has special physical and chemical properties that plays a pivotal role in in hundreds of products .
Its most uses is in alloy production _ specially alloys with chromium and other metals to produce stainless steel and heat – resistant steels .
About 69 % of this element is used to make stainless steel . other 15 % is used in steel and non-ferrous alloys ; specially in highly industrial , aerospace and military applications . About 8% in plating and other 3% in casting .
About 3% is used in batteries used in electronic components and in batteries used in portable equipment hybrid vehicle , and approximately 2 % of it is devoted to the use of chemicals , catalyst and dyeing materials .
Nickel is easily combined with most metals such as copper , chromium , iron and Molybdenum . The addition to other metals changes the properties of the resulting alloy and can be used to generate the desired properties such as corrosion improvement or resistance to oxidation , increasing efficiency at high temperature or lower coefficients of thermal expansion .

● There are some properties of nickel mentioned below :
- High mechanical properties and excellent resistance against corrosive environments
- Maintaining strength at a high temperature
- Suitable ductility and toughness against low temperatures
- Easy mixture with sulfur and arsenic
- High durability against climate conditions and not to oxide
Most of nickel obtained are derived from two types of mine : brick-colored soils , the most important source of nickel ore , and the existing sulfide on terrestrial magma . There are two types of nickel minerals :
1) Sulfide
Sulfide occupies two - thirds of the world's consumption . These minerals are converted to nickel oxide with the help of flotation and filtration and then settles and refined .
2) Oxide
Nickel oxide is also refined with the aim of hydrometallurgy refining ( like washing with ammonia . )
- Coinage
- Construction of chemical instruments in special alloys
- Components of machinery
- Use of nickel chromium coating for food industries
- Fixing and shaping of electric components due to the hardness and strength of nickel
- Components of rocket and aerospace
- Chemical industrial plumbing
- Creation of stainless steel and alloy steel
- Rechargeable batteries
- Casting an products
- Kitchen instruments
- Drilling
- Steel production industries in order to produce washing machines and utensils .
The world's nickel resources are currently estimated at around 300 million tons . Australia , Indonesia , South Africa , Russia and Canada have allocated more than 50 % of the world's global nickel resources . Despite the fact that approximately 80 % of the total nickel has been mined historically during the past three decades , it has also been added to its reserves and resources .
Different parameters contribute to this evolution such as gathering information about new resources and advanced technologies in mining .
Therefore , the degradation of nickel ores is not necessarily a sign of a reduction of resources . It reflects the innovation and the improvements made in mining and processing technologies .
It is also estimated that there is considerable reserves of this element in the depths of oceans . Bulges of manganese , which are observed in the depths of oceans , contain significant quantities of nickel .
Recent estimates suggest there are more than 290 million tons of nickel in the reserves . The development of mining technologies in the depths of oceans is predicted to facilitate access to these resources in the future .
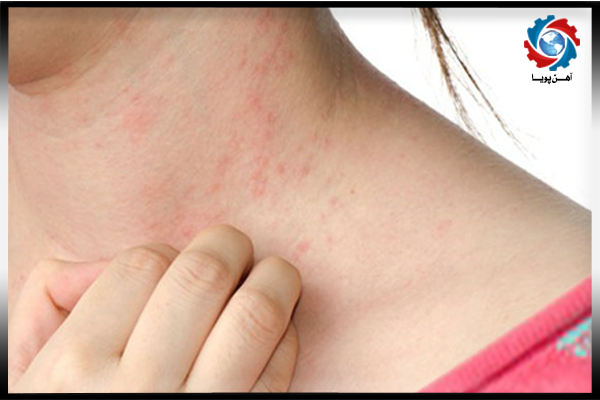
Carbon sulfide vapour is probably carcinogens that need to be careful when using it . Carbonic nickel is a highly toxic gas . Nickel contact with sensitive people skin may cause allergies .

Ahan Pouya with more than a decade of best-selling experience, adheres to professional and ethical principles in the field of selling and buying at inside and outside the borders of Iran, helping you in the steel industry.