
Alloys are actually the combination of some metals or the combination of one metal with another impure element .
French word aloi which comes from old French alei , and the Latin word alligare are the roots of the word alloy in modern English today . Amalgam , admixture , combination and some others are synonyms for alloys . Now it is the time to find out what alloys really are ?
Alloys are actually the combination of some metals or the combination of one metal with another impure element . Alloys are metallic materials that have combined with other substances .
To provide an alloy at least two chemical substances are needed . One of which should be metal . This metal is called primary metal or thebase metal . The name of this metal is the name of resulting alloy itself .
The metal that is usually soft ( like Aluminium ) may combine with another malleable metal such as copper .
Although two metals are very ductile but the ending alloy will have more strength . Augmenting a small amountof carbon to iron creates the alloy called steel . Steel is one of the most common and useful alloys in modernworld . By adding chromium to steel the strength will enhance and stainlesssteel will obtain .
- Resistance
- Firmness
- Decay
- Magnetizability
- Moldability
- Machinability
The main material of alloys must be a metallic substance but the other elements could be non-metallic elements . For example steel( or silicon steel ) creates by the combination of iron with non-metallic carbon . The result mixture often has other characteristics which are different from the main metals . For example hardness and strength are two factors that can increase by making alloys .
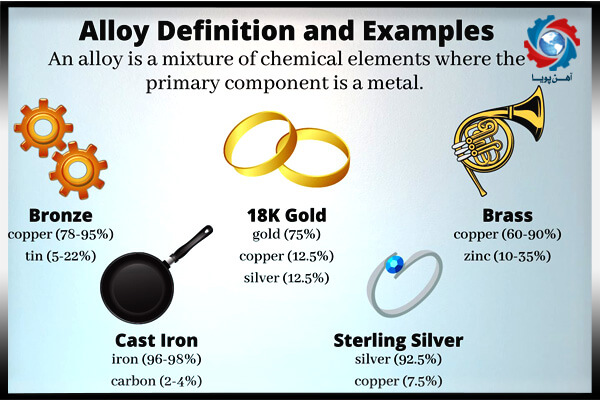
For example combination of copper and gold creates red gold . Combination of red gold and silver creates white gold and combination of copper and silver produces sterling silver . Sterling silver has 92.5 & of silver and 7.5 % of other metals in it .
The most common way to mix the metals is meling them , mixing them and at the end letting them cool down .
However some substances that may contain metallic bases like Aluminium oxide ( sapphire ) or beryllium aluminium silicate ( emerald ) or sodium chloride ( salt ) but they don’t behave like metals .Then alloys often keep the characteristics of primary metals in resulting alloys . These characteristics are incliding : electricalconductivity, ductility, opacity, and luster .
Electrical conductivity refers to how well a metal acts with electricity currents .
electromagnetics or other radiations And luster is defined how a light interacts with the surface of our metal .
Humans discovered the importance of alloys in very ancient times . They mixed copper and zinc to create brass and copper and tin to create bronze .
Todays , the most important alloy is alloy steels which have made up of elements like carbon with iron . Someother elements can create alloying steel such as chromium , nickel , manganese , molybdenum , silicon , tungsten, vanadium , and boron .
Alloys ae used in very of applications in different fields . They are used because the chemical and mechanical properties of them have increased .
Nonferrous alloys are mostly used in coinage. For example : copper – nickel , bronze , and aluminum .
Steel alloys used in different things like surgical tools , automobiles and buildings .
Titanium alloys used in the aerospace industry . Beryllium-copper alloys used in non-sparking tools .

Fusible metlals or fusible alloys are actually metals that have meting point below 232 °c ( tin melting point ) .
These substances are mostly the combination of metals that by themselves have the low melting points . Fusible alloys have more application in solder workings .
The 2nd application of fusible alloys is in fuses when fuses becomes too much . The alloys interrupts theelectrical flow for better safety .
Many fusible alloys have created today to melt at 90-100 °c . For example Darcet’s alloy and Wood’s alloy . The primary melts at 98°c and the 20d melts at about 70°c .
It is interesting to know that there are metal supermarkets across the U.S, Canada and U.K . They have been built in 1985 . Metal professionals are there and they provide metallic products and customer services though .
Products including : tubes , sheets , plates and bars .
A molten metal like oil or water will not mix with all of the elements . For example sheer iron will not mix with the pure copper . Another key point is that even when two elements are soluble , each of them has a saturation point . Saturation point is a point in which the substance , gases , liquids or solids becomes insatiable . Beyond this point no more components could be added .
For example iron can not hold more than about 7% of carbon in it . However the elements of an alloy must solve in liquid state but they may not solve in solid state . If the solution remains in the solid mood , the alloyforms a solid solution . The solid solution has integrated crystal order called phase .
As the alloy cools down it becomes insoluble and may consist of different kinds of crystals which creates microstructure of phases .
Meteorites is the naturally occurred combination of iron and nickel . Another example of natural alloys is Electrum which is an alloy of silver and gold .
We do use electrum in different applications such as in drinking vessels , to make jewelry and in coinage .
Bronze was one of the first alloys that humans made . Bronze is mixture of tin and copper which has numerous applications such as in ship and boat fitting , and in sculptury.
Strength of bronze was clear for our ancients . They knew its strength is much more than its components itself . They have been using bronze in fighting tools too .
Steel was another common alloy that suddenly discovered by just fuming the iron ores in factories of iron .Pewter , brassand pig ironare also some of the other ancient alloys . Pewter has a variety of applications suchas for dishes , church vessels and decorative items . Pig iron was used in pudding furnaces and recently in steelmaking . Nowadays brass is also widely used in sanitary plumbing fixtures , locksmithing and in musicalinstruments .

The mixture of chemical components which at least has a metallic element , is called alloy . Alloys may be made up of iron or other metals .
The importance of alloys is clear for humans from ancient times up to now . Importance of alloys is because the toughness and hardness of alloys are much more on comparison with pure metals .
Alloys has so many applications in different fields from sculpture making to making surgical tools and many others .

Ahan Pouya with more than a decade of best-selling experience, adheres to professional and ethical principles in the field of selling and buying at inside and outside the borders of Iran, helping you in the steel industry.